Therapeutic equivalence evaluations in this publication are not official fda actions affecting the legal status of products under the act. see note 5, supra. 1 generic substitution laws are state specific and many require use of the orange book as a reference.


Professional care and judgment should be exercised in using the orange book.



Orange book pharmacy ab rating. First, if you have the trade name, search the electronic orange book's rx or otc section using the proprietary name search. In july 2014, fda granted the ab rating to perrigo’s 1% testosterone gel drug product (nda 203098) approved in january 2013, and a bx rating to teva’s (nda 202763) approved in february 2012. The orange book is an important publication published by the fda that serves as the gold standard reference for generic drug substitution.
Facility’s color rating to orange or red and that may be escalated for possible investigation or supplier engagement. Approved drug products with therapeutic equivalence evaluations. Then use the ingredient search for.
With respect to the dilemma concerning cardizem cd noted earlier, a search of the orange book revealed that cardizem cd 240 mg is rated “ab3”; Depakote is available in both a delayed release and extended release formulation among others and, just as with bupropion, it is best not to assume that all patients follow the typical dosing (ie once daily for extended release and twice daily for delayed. One of the compact disc standards collections in the rainbow books series.
When the first generic concerta products came to market late in 2013, they were orange book ab rated to the brand name. Builds on the previous orange book to help improve risk management further and to embed this as a routine part of how we operate. This means they were classified as bioequivalent by the fda and therefore, were substitutable with one another.
Fda recently granted a therapeutic equivalence (te) rating of ab to a “generic” version of an innovator product approved via a 505b2 nda. Fda’s approved drug products with therapeutic equivalence evaluations (orange book) identifies drug products approved on the basis of safety and effectiveness. Fda’s orange book and ab ratings of pharmaceutical drug products:
Public sector organisations cannot be risk averse and be successful. This determines the ingredient (s). Evaluations of therapeutic equivalence for prescription drugs are based on scientific and medical evaluations by fda.
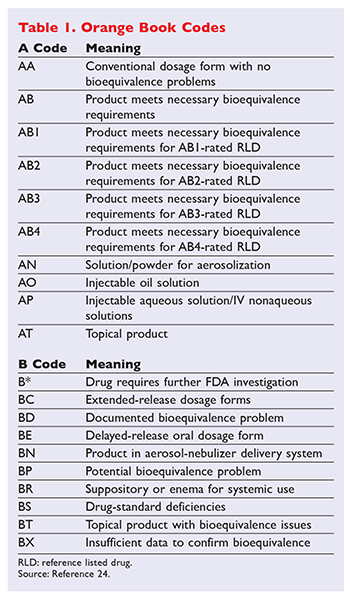
The two generic products now have a therapeutic equivalence rating of bx in fda’s orange book: Codes beginning with ‘a’ signify the product is deemed therapeutically equivalent to the reference product for the category. Cdr kendra stewart, r.ph., pharm.d.
Orange book equivalency rating of extended release methylphenidate products manufactured by mallinkrodt and kudco from “ab” to “bx” due to concerns about bioavailability equivalency with janssen pharmaceuticals’ concerta product. Vijay m kale, assistant professor of Each are versions of abbvie’s androgel® and employed 505(b)(2).
Zestril 30 mg is the only product available. More details concerning fda’s action, the reasons for it, and the consequences are found here: A guide to community pharmacist.
Office of generic drugs policy center for drug evaluation & research u.s. National association of boards of pharmacy. Fda’s orange book and ab ratings of pharmaceutical drug products:
On march 23, 2020, fda removed from the orange book the listings for biological products that have been approved in applications under section 505 of the fd&c act because these products are no longer listed drugs (see section 7002 (e) (4) of the biologics price. Approved drug products with therapeutic equivalence evaluations. That rating can be useful in determining what is used in product selection, but it is not necessarily determinative.
Thus, generic products rated “ab3”, such as apotex’s generic, are suitable for substitution. Such a rating means that the available data on those generic drug products are insufficient for fda to determine therapeutic equivalence with concerta, marketed by janssen. The north carolina product selection law does not refer to the orange book rating published by the food and drug administration.
The iupac compendium of analytical nomenclature informally known as the orange book. The orange book has been revised. The full publication title is approved drug products with therapeutic equivalence evaluations, but it is commonly known as the orange book.
Must a drug be rated ab in fda’s orange book to be used in product selection in north carolina?